NEWS
CDC Recommends Updated Covid-19 Boosters For Everyone Ages 6 Months and Older
Published
8 months agoon
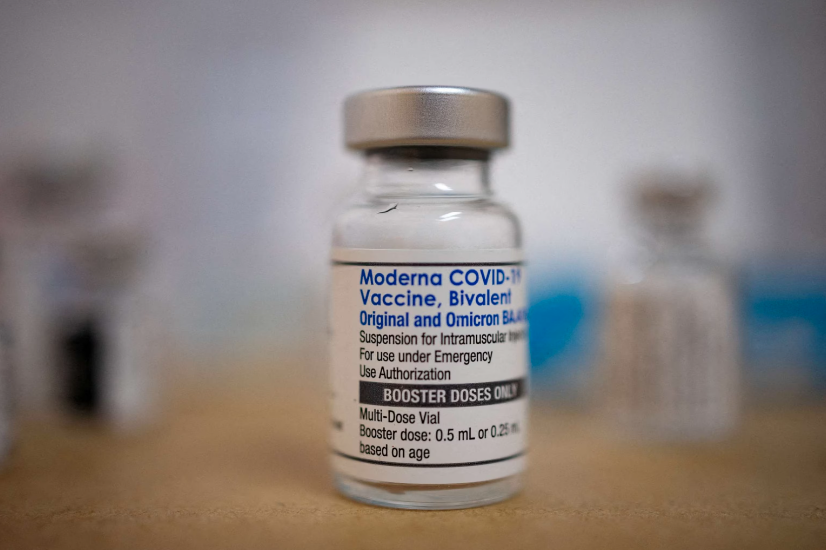
Courtesy of Skippack Pharmacy
The Centers for Disease Control and Prevention on Tuesday recommended that everyone ages 6 months and older get an updated COVID-19 booster vaccine, which will be available this week.
The U.S. Food and Drug Administration on Monday announced the approval of updated COVID-19 boosters from Pfizer and Moderna for emergency use. A CDC advisory committee met Tuesday to determine eligibility guidelines.
“We have more tools than ever to prevent the worst outcomes from COVID-19. CDC is now recommending updated COVID-19 vaccination for everyone 6 months and older to better protect you and your loved ones,” CDC Director Mandy Cohen said in a statement.
“Vaccination remains critical to public health and continued protection against serious consequences of COVID-19, including hospitalization and death,” said Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research. “The public can be assured that these updated vaccines have met the agency’s rigorous scientific standards for safety, effectiveness, and manufacturing quality. We very much encourage those who are eligible to consider getting vaccinated.”
According to the FDA, anyone age 5 or older regardless of vaccination history can receive a booster shot at least two months after a previous vaccination.
Those 6 months to 4 years old who were previously vaccinated are eligible to receive one or two doses of the updated booster, depending on which vaccine they received. Unvaccinated individuals ages 6 months to 4 years are eligible to receive three doses of the updated booster.
The updated monovalent vaccine targets the XBB.1.5 omicron subvariant and related lineages, but vaccine manufacturers say it will offer protection against all variants currently in circulation.
Moderna said its updated vaccine protects against newer variants including the latest omicron subvariant, BA.2.86, nicknamed “Pirola.”
Pfizer, meanwhile, said in a recent statement that pre-clinical data show its updated Omicron XBB.1.5-adapted vaccine “generates an improved response against multiple XBB-related sublineages, including XBB.1.5, XBB.1.16, XBB.2.3, and EG.5.1 (Eris).”
An updated booster produced by Novavax is still awaiting FDA approval. The Novavax shot does not use mRNA technology, unlike those from Moderna and Pfizer.
“Unless there is a sudden spike in deaths or some drastic negative shift in the effects of COVID, the U.S. government and medical industry are going to have a difficult time convincing Americans to treat COVID as anything more than the common flu,” said strategic communications consultant Robbie Vorhaus, founder and CEO of Vorhaus Communications, Inc.
“Americans are COVID-weary weary, and hearing any communications aimed at convincing them to get a booster immunization is only treated as a nuisance,” Vorhaus said. “If the U.S. government genuinely wants to convince Americans to get their COVID booster, it must change its messaging from fear-based and clinical to something that inspires community and living longer and better.”
According to the CDC, COVID hospitalizations have been climbing in recent weeks, and are likely to continue increasing in the coming weeks.
The agency reported 18,871 hospital admissions for COVID-19 infections in the week ending Sept. 2, the last week for which data were available, up 8.7% from the week before, following weeks of double-digit increases. The agency reported that deaths due to COVID-19 were up 10.5% from the week before.
In the latest forecast published Sept. 4, the agency predicted that hospital admissions will increase, with potentially up to 9,100 daily admissions reported on Oct. 9.
TMX contributed to this article.
More From Lifestylogy
-


Reunited – Cat Gets in Amazon Box, Accidentally Shipped from…
-
Professional Name Consultant Shares Predictions For Top Name Choices For…
-


Billie Eilish Appears To Be Teasing New Music As Billboards…
-


Vanderpump Rules’ Ariana Madix – 1st Broadway Bow for ‘Chicago’…
-


CosMc’s Appears In Illinois Prompting McDonalds Fans To Think Coffee…
-


Taylor Swift Introduces Her Eras Tour Movie To Theater Full…
-


Aldi Customer Tweets Picture Of Comically Misshapen Marshmallow Bunnies &…
-


Taco Bell Manager Rescues Baby that Stopped Breathing at Drive-Through…
-


Taylor Swift Gives Hat to Stunned Fan. Also Says Thanks,…
